Quantum Frostbite
How lasers make things very very cold
Cartoons and common sense have made us all think that lasers can only heat things up, but it turns out that some of the lowest temperatures ever achieved have used lasers to cool things down. Today, I’m going to try and explain this unintuitive phenomenon from the ground up (more or less).
What is a Laser?
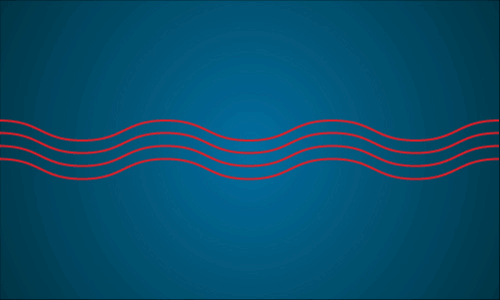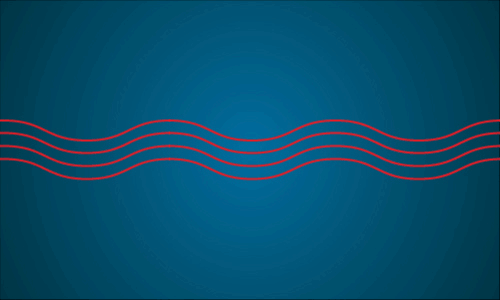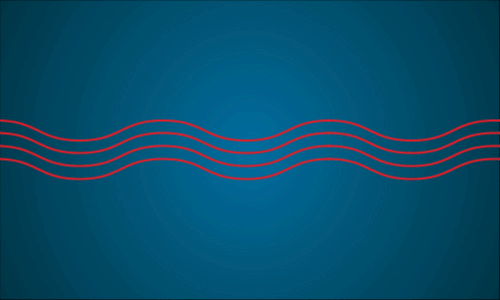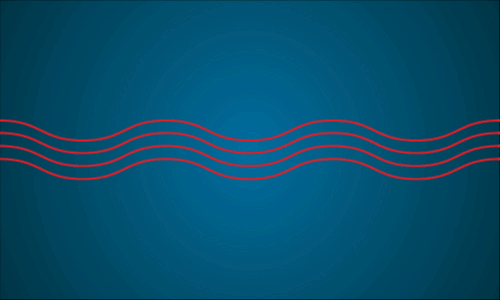
Light comes in many wavelengths, whether it’s infrared, visible light or even x-rays. Lasers are specifically sources of monochromatic light, meaning all light in a laser beam is of the same wavelength (’colour’). They are endlessly useful in many ways such as: carrying TV signals, printers, spectrometry, DVD players and precision tools.
Figure 1: Laser light waves, all of the same wavelength with peaks lined up (in phase).
What Does it Mean to be Cool?
Now I know that in every other context I am unequipped to tell you what it means to be cool, but here I’ve found the one place where I’m qualified. Thermodynamics.
You may vaguely remember from school science classes that hotter molecules have more energy and therefore move more quickly. This is important as the methods discussed in this article use the mechanical properties of light to slow stuff down, cooling it. To put this into digestible terms, molecules and atoms in the air at room temperature are moving at roughly the speed of sound. Velocity scales with the square root of temperature.
In day to day life (unless you’re a silly American), we use the Celsius scale to measure scale to measure temperature where the freezing and boiling point of water is set to 0 and 100 degrees respectively. This is a relative scale. Scientists use the Kelvin scale, which starts at absolute zero (0K), the temperature where all motion ceases. According to the third law of thermodynamics, this is impossible to reach exactly but we can get close! 0K is equivalent to -273.15°C.
Laser Cooling of Atomic Beams via Doppler Method
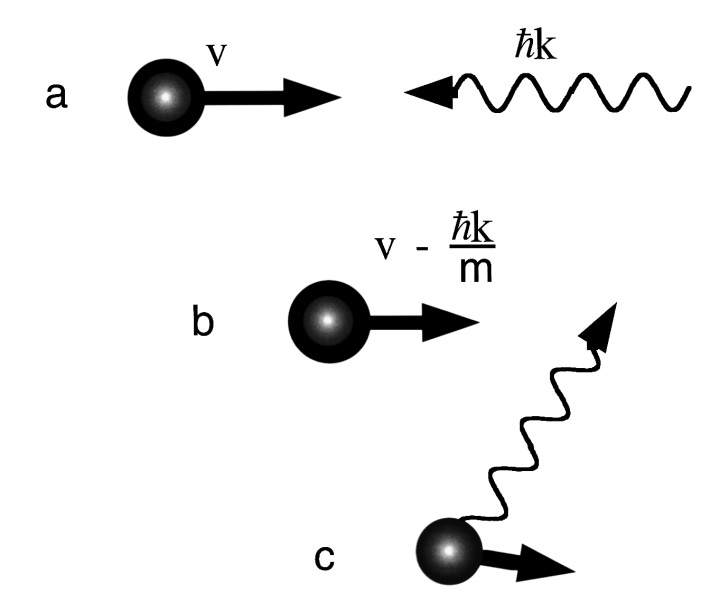
An atomic beam can be slowed down by using the transfer of momentum that occurs when an atom absorbs a photon. When an atom absorbs an incoming photon, if it is energetic enough then the atom will move to a higher energy level. This excited state of the atom is unstable and will revert back to its original (ground) state energy level by emitting a photon in a random direction. As you can imagine, the emitted photon has momentum and so the main key is to ensure that atoms emit photons in a direction opposite to motion when returning to the ground state energy, slowing it down and by extension, cooling it.
Figure 1: (a) An atom with velocity v encounters a photon with momentum h/λ (Planck’s constant/wavelength), (b) after absorbing the photon, the atom is slowed by
where k is the wavenumber of the photon and m is the atom’s mass, (c) after re-radiation in a random direction, on average the atom is slower than in (a).
An atom travelling towards a light source will ‘see’ that source as blue-shifted (higher energy) and an atom travelling away from the light source will be red-shifted (lower energy). This is due to the doppler effect (Figure 2).
Figure 2: The doppler effect of sound waves in 1D. Imagine the difference between the waves that would reach you at (0, -10) compared to (0, 10). For those who prefer analogies, think of the difference in how often waves will hit you if you 1) stand still in the sea next to the shore compared to 2) running into the sea.
When calibrating cooling lasers, frequency of laser light is generally tuned to a slightly lower frequency that the atoms would like to absorb (comment for more info) so that it is more likely that the each atom is more likely to absorb a photon that it is moving towards (that’ll slow it down and cool it) rather than one that it is moving away from (that’ll speed it up and heat it).
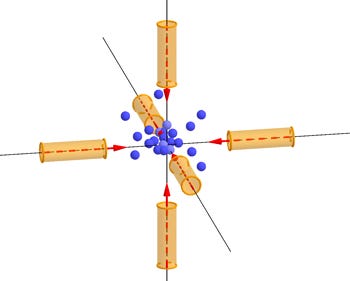
Figure 3: Graphic of atoms trapped in 3D by optical molasses created by 6 laser beams.
What Can we do with Cool Atoms?
Atomic Clocks
Timekeeping is important in many facets of life and science. Atomic clocks are ridiculously accurate devices that can be used mundanely to improve the quality of GPS, or to help in general relativity and dark matter experiments. They are especially useful for space navigation as the most advanced atomic clocks would take 30 billion years to even lose a second. This is useful in long space missions where fractions of seconds being lost by a fast moving spacecraft can result in distance errors in calculation. If we were to communicate with a spacecraft that lost a third of a second of time, this would create a 100km distance error.
Figure 4: Atomic Clock art produced by NASA
Atomic clocks work by measuring the frequency of photon emission of (traditionally) Caesium (Cs). The atoms used for timekeeping need to be incredibly cold (and therefore slow) in order to minimise collisions that create measurement errors.
Quantum Computing
Quantum computing uses ‘qubits’ that are subatomic particles allowed to exist in more than one state. In the context is classic binary, a qubit could both have the state of a 0 and a 1 simultaneously. Classical computers can only encode streams of electrical impulses in binary and therefore have a comparatively limited processing power. Let me know in the comments if you’d like a more dedicated post on quantum computing and the quantum features that govern it, namely Superposition, Entanglement and Decoherence.
Figure 5: A quantum computer
A quantum processor is about the same size as a regular one found in a laptop but the hardware system is as big as a Range Rover, with most of that made up of cooling systems. See quantum computing systems need to be not much more than a hundredth of a Kelvin above absolute zero so that the processors’ retain their states and don’t decay.
Ok that’s all I’m bothered to talk about now but it’s no surprise that many ultracold atom research groups are around in universities, and the engineering and theory work to harness quantum effects for our use will lead to many technological improvements in the future.
References
The Nobel Prize in Physics 1997. NobelPrize.org. Nobel Prize Outreach AB 2023. Wed. 20 Sep 2023.
IBM quantum, 2023
Nobel Lecture: Laser cooling and trapping of neutral atoms, William D. Phillips, 1998
www.nasa.gov
Socials:
Instagram: mario.k.n
The website formerly known as Twitter: marioknicola
Email: marioknicola@gmail.com
A song I’m listening to:




What a delightful read
I would also love to hear you break down the principles of quantum computing